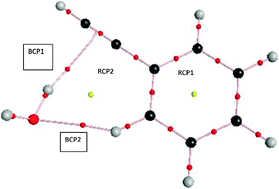
Rotational spectra of five isotopologues of the title complex, C6H5CCH⋯H2O, C6H5CCH⋯HOD, C6H5CCH⋯D2O, C6H5CCH⋯H218O and C6H5CCD⋯H2O, were measured and analyzed. The parent isotopologue is an asymmetric top with κ = −0.73. The complex is effectively planar (ab inertial plane) and both ‘a’ and ‘b’ dipole transitions have been observed but no c dipole transition could be seen. All the transitions of the parent complex are split into two resulting from an internal motion interchanging the two H atoms in H2O. This is confirmed by the absence of such doubling for the C6H5CCH⋯HOD complex and a significant reduction in the splitting for the D2O analog. The rotational spectra, unambiguously, reveal a structure in which H2O has both O–H⋯π (π cloud of acetylene moiety) and C–H⋯O (ortho C–H group of phenylacetylene) interactions. This is in agreement with the structure deduced by IR-UV double resonance studies (Singh et al., J. Phys. Chem. A, 2008, 112, 3360) and also with the global minimum predicted by advanced electronic structure theory calculations (Sedlack et al., J. Phys. Chem. A, 2009, 113, 6620). Atoms in Molecule (AIM) theoretical analysis of the complex reveals the presence of both O–H⋯π and C–H⋯O hydrogen bonds. More interestingly, based on the electron densities at the bond critical points, this analysis suggests that both these interactions are equally strong. Moreover, the presence of both these interactions leads to significant deviation from linearity of both hydrogen bonds.
 Please wait while we load your content...
Please wait while we load your content...