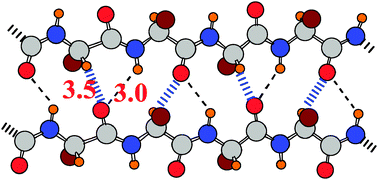
Whereas CH⋯O H-bonds are usually weaker than interpeptide NH⋯O H-bonds, this is not necessarily the case within proteins. The nominally weaker CH⋯O are surprisingly strong, comparable to, and in some cases stronger than, the NH⋯O H-bonds in the context of the forces that hold together the adjacent strands in protein β-sheets. The peptide NH is greatly weakened as proton donor in certain conformations of the protein backbone, particularly extended structures, and forms correspondingly weaker H-bonds. The PH group is a weak proton donor, but will form PH⋯N H-bonds. However, there is a stronger interaction in which P can engage, in which the P atom, not the H, directly approaches the N electron donor to establish a direct P⋯N interaction. This approach is stabilized by the same sort of electron transfer from the N lone pair to the P–H σ* antibond that characterizes the PH⋯N H-bond.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...