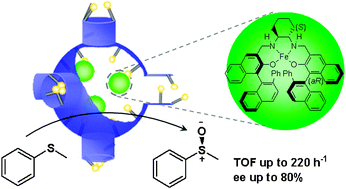
The solid catalysts for asymmetric oxidation of sulfides were prepared by encapsulating a chiral iron salan complex [Fe(salan)] in the nanocages of mesoporous silicas. The microenvironment of nanocages was finely tuned using silylation reagents with different kinds of organic groups, such as propyl (C3), 1-butyl-3-propyl-4,5-dihydroimidazolium bromide (ILBr), N-propyl-N,N,N-tri-n-butylammonium chloride (TBNCl) and N-propyl-N,N,N-tri-n-butylammonium bromide (TBNBr), and investigated by water and benzene adsorption. Fe(salan) encapsulated in the amphiphilic nanocage shows much higher enantioselectivity and activity than that in hydrophobic or hydrophilic nanocage for the asymmetric oxidation of thioanisole using H2O2 as oxidant. The TOF of Fe(salan) encapsulated in the nanocage modified with TBNBr can reach as high as 220 h−1, even higher than homogeneous Fe(salan) with a TOF of 112 h−1. The enhanced catalytic activity is mainly due to the fast diffusion of H2O2 and sulfide in the amphiphilic nanocage. The above results suggest that the microenvironment modification of the nanocage is an efficient method to synthesize highly efficient solid catalysts for asymmetric catalysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...