Free energy evaluation of the p53-Mdm2 complex from unbinding work measured by dynamic force spectroscopy
Abstract
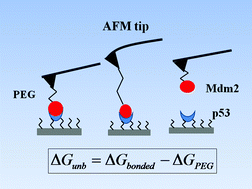
The complex between the tumor suppressor p53 and its down-regulator Mdm2 has been studied by
- This article is part of the themed collection: Biophysics and biophysical chemistry in PCCP

 Please wait while we load your content...
Please wait while we load your content...