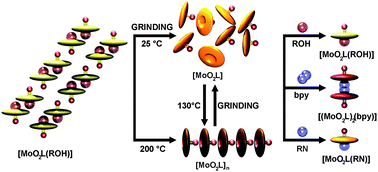
This article describes the selective cleavage of coordination bonds by mechanochemical methods and the further application of the thus obtained precursors for a facile preparation of new coordination compounds. In the class of dioxomolybdenum(VI) coordination compounds, [MoO2L(ROH)], where L stands for a tridentate dianionic ONO ligand and ROH represents different alcohol molecules, mechanical treatment induces an exclusive cleavage of the molybdenum–alcohol bond, which can thus be considered as a mechanosensitive bond. Alcohol removal can also be accomplished by heating. Both grinding and heating resulted in highly reactive, coordinatively unsaturated compounds, an orange amorphous pentacoordinated [MoO2L] (I) and the brown polymeric [MoO2L]n ((I)nnn), respectively. Even though both I and (I)nn are stable at room temperature, they can be interconverted using only solvent-free techniques, a conversion followed by a colour change of the sample. The tendency of such unsaturated complexes to complete their coordination spheres was exploited for the efficient solution and solvent-free syntheses of octahedral molybdenum complexes with selected O- and N-donating ligands. Both approaches herein used, solution and solvent-free, have proved to be superior under specific conditions and their respective advantages and weaknesses are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...