Intramolecular monomer-on-monomer (MoM) Mitsunobu cyclization for the synthesis of benzofused thiadiazepine-dioxides†
Abstract
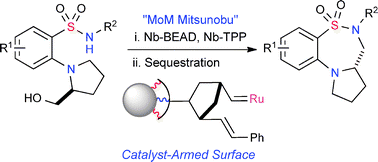
The utilization of a monomer-on-monomer (MoM) intramolecular Mitsunobu cyclization reaction employing norbornenyl-tagged (Nb-tagged) reagents is reported for the synthesis of benzofused thiadiazepine-dioxides. Facile

 Please wait while we load your content...
Please wait while we load your content...