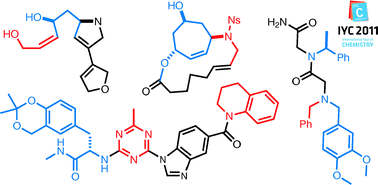
Combinatorial chemistry provides a powerful tool for the rapid creation of large numbers of synthetic compounds. Ideally, these libraries should be a rich source of bioactive molecules, but there is the general feeling that the initial promise of combinatorial chemistry has not yet been realized. In particular, enthusiasm for conducting unbiased (non-structure-guided) screens of large libraries for protein or RNA ligands has waned. A central challenge in this area is to devise methods for the synthesis of chemically diverse, high-quality libraries of molecules with many of the desirable features of natural products. These include diverse functionality, a significant representation of chiral sp3 centers that provide conformational bias to the molecule, significant skeletal diversity, and good pharmacokinetic properties. However, these libraries must be easy to make from cheap, readily available building blocks, ideally those that would support convenient hit optimization/structure reactivity relationship studies. Meeting these challenges will not be easy. Here I review some recent advances in this area and provide some thoughts on likely important developments in the next few years.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...