Nickel-catalyzed reductive cleavage of aryl–oxygen bonds in alkoxy- and pivaloxyarenes using hydrosilanes as a mild reducing agent†
Abstract
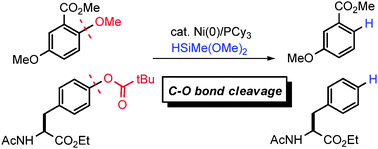
A nickel-catalyzed reductive deoxygenation of aryl alkyl ethers and aryl pivalates has been developed. Hydrosilanes serve as a mild

 Please wait while we load your content...
Please wait while we load your content...