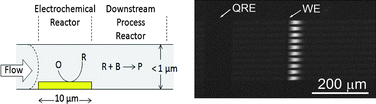
In situ generation of reactive species within confined geometries, such as nanopores or nanochannels is of significant interest in overcoming mass transport limitations in chemical reactivity. Solvent electrolysis is a simple process that can readily be coupled to nanochannels for the electrochemical generation of reactive species, such as H2. Here the production of hydrogen-rich liquid volumes within nanofluidic structures, without bubble nucleation or nanochannel occlusion, is explored both experimentally and by modeling. Devices comprised of multiple horizontal nanochannels intersecting planar working and quasi-reference electrodes were constructed and used to study the effects of confinement and reduced working volume on the electrochemical reduction of H2O to H2 and OH−. H2 production in the nanochannel-embedded electrode reactor output was monitored by fluorescence emission of fluorescein, which exhibits a pH-dependent emission intensity. Initially, the fluorescein solution was buffered to pH 6.0 prior to stepping the potential cathodic of E0′ for the generation of OH− and H2. Because the electrochemical products are obtained in a 2 : 1 stoichiometry, local measurements of pH during and after the cathodic potential steps can be converted into H2 production rates. Independent experimental estimates of the local H2 concentration were then obtained from the spatiotemporal fluorescence behavior and current measurements, and these were compared with finite element simulations accounting for electrolysis and subsequent convection and diffusion within the confined geometry. Local dissolved H2 concentrations were correlated to partial pressures through Henry's Law and values as large as 8.3 atm were obtained at the most negative potential steps. The downstream availability of electrolytically produced H2 in nanochannels is evaluated in terms of its possible use as a downstream reducing reagent. The results obtained here indicate that H2 can easily reach saturation concentrations at modest overpotentials.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...