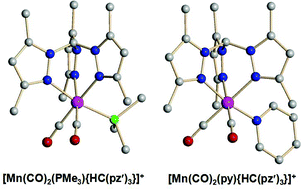
The tricarbonyl [Mn(CO)3{HC(pz′)3}][PF6] 1+[PF6]− (pz′ = 3,5-dimethylpyrazolyl) reacts with a range of P-, N- and C-donor ligands, L, in the presence of trimethylamine oxide to give [Mn(CO)2L{HC(pz′)3}]+ {L = PEt33+, P(OEt)34+, P(OCH2)3CEt 5+, py 6+, MeCN 7+, CNBut8+ and CNXyl 9+}. The complex [Mn(CO)2(PMe3){HC(pz′)3}]+2+ is formed by reaction of 7+ with PMe3. Complexes 2+ and 6+ were structurally characterised by X-ray diffraction methods. Reaction of 7+ with half a molar equivalent of 4,4′-bipyridine gives a purple compound assumed to be the bridged dimer [{HC(pz′)3}Mn(CO)2(μ-4,4′-bipy)Mn(CO)2{HC(pz′)3}]2+102+. The relative electron donating ability of HC(pz′)3 has been established by comparison with the cyclopentadienyl and tris(pyrazolyl)borate analogues. Cyclic voltammetry shows that each of the complexes undergoes an irreversible oxidation. The correlation between the average carbonyl stretching frequency and the oxidation potential for complexes of P- and C-donor ligands is coincident with the correlation observed for [Mn(CO)3−mLm(η-C5H5−nMen)]. The data for complexes of N-donor ligands, however, are not coincident due to the presence of a node (and phase change) between the metal and the N-donor in the HOMO of the complex as suggested by preliminary DFT calculations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...