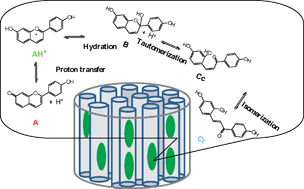
We report a spectroscopic study of the network of reactions of a flavylium dye encapsulated in the one-dimensional channels of zeolite L. The positively charged 7,4′-dihydroxyflavylium (AH+) is easily incorporated and remains stable in zeolite L channels. Once encapsulated, the flavylium exhibits a red shift in the excitation spectrum comparative to aqueous solutions. Moreover, contrary to the observed behavior in water, no excited state proton transfer takes place in the loaded crystals, corroborating the encapsulation of AH+. The trans-chalcone (Ct) form from the same flavylium network could also be encapsulated inside the zeolite L, using toluene with 20% triethylamine as solvent and K+ as counter ion of the negative framework of the zeolite. The encapsulation of Ct is confirmed by changes on the excitation spectrum and by a blue shift in the emission. The encapsulated Ct was shown to generate AH+ when the Ct-loaded crystals were suspended in water, which proves that isomerization, tautomerization and dehydration reactions take place inside the zeolite L.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...