Towards mRNA with superior translational activity: synthesis and properties of ARCA tetraphosphates with single phosphorothioate modifications†‡
Abstract
We describe the chemical synthesis and preliminary biophysical and biochemical characterization of a series of
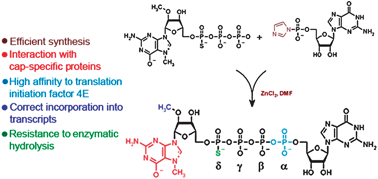
- This article is part of the themed collections: Biophosphates and mRNA vaccines against COVID-19: Celebrating the 2023 Nobel Prize in Physiology or Medicine

 Please wait while we load your content...
Please wait while we load your content...