Ordered mesoporous silicananoparticles with and without embedded iron oxide nanoparticles: structure evolution during synthesis
Abstract
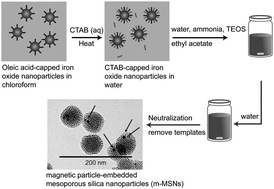
This work reports on the structural evolution during room temperature synthesis of hexagonally ordered mesoporous

 Please wait while we load your content...
Please wait while we load your content...