Cation ordering in perovskites
Abstract
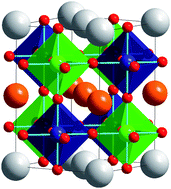
Although both A- and B-site cations have the same simple cubic topology in the perovskite structure they typically adopt different patterns of chemical order. As a general rule B-site cations order more readily than A-site cations. When cation ordering does occur, rock

 Please wait while we load your content...
Please wait while we load your content...