A facile, protic ionic liquid route to N-substituted 5-hydroxy-4-methyl-3-oxoisoindoline-1-carboxamides and N-substituted 3-oxoisoindoline-4-carboxylic acids
Abstract
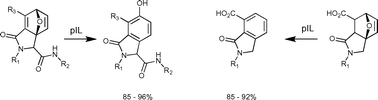
Treatment of highly decorated bicyclo[2.2.1]heptadienes with the protic ionic liquid,

 Please wait while we load your content...
Please wait while we load your content...