Intermediate temperature solid oxide electrolysis cell using LaGaO3 based perovskite electrolyte
Abstract
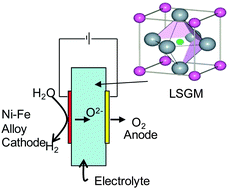
The application of a LaGaO3 based electrolyte for steam electrolysis was studied and it was found that the H2 formation rate obeys the Faraday's law suggesting unity of

 Please wait while we load your content...
Please wait while we load your content...