Rapid assembly of explicit, functional silicones†
Abstract
The impressive surface activity of silicones can be enhanced by the incorporation of hydrophilic organic functional
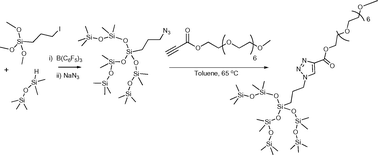
- This article is part of the themed collection: New horizon of organosilicon chemistry

 Please wait while we load your content...
Please wait while we load your content...