Dimeric versus polymeric coordination in copper(ii) cationic complexes with bis(chelating) oxime and amide ligands†
Abstract
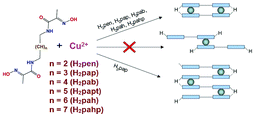
A series of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O–H⋯O–N
N–O–H⋯O–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) between cis-arranged oximato-
between cis-arranged oximato-
* Corresponding authors
a
Department of Chemistry, National Taras Shevchenko University, Kiev, Ukraine
E-mail:
ifritsky@univ.kiev.ua
Fax: +38 044 239 33 93
Tel: +38 044 239 33 93
b
Faculty of Chemistry, University of Wroclaw, F. Joliot-Curie 14, Wroclaw, Poland
E-mail:
kontecka@wchuwr.pl
Fax: +48 71 3757342
Tel: +48 71 3757342
c Department of Chemistry, University of Joensuu, P.O.Box 111, Joensuu, Finland
A series of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O–H⋯O–N
N–O–H⋯O–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) between cis-arranged oximato-
between cis-arranged oximato-

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. I. Buvailo, E. Gumienna-Kontecka, S. V. Pavlova, I. O. Fritsky and M. Haukka, Dalton Trans., 2010, 39, 6266 DOI: 10.1039/C0DT00008F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
