Catalytic oxygenation of phenols by arthropod hemocyanin, an oxygen carrier protein, from Portunus trituberculatus†
Abstract
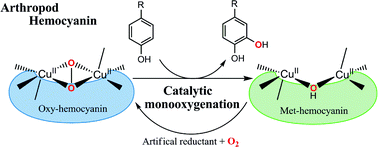
The hexamer (Pt-6Hc) of swimming crab Portunus trituberculatus hemocyanin (Pt-Hc) and one of its monomeric subunits (Pt-1Hc) have been purified and converted to an efficient
- This article is part of the themed collection: Bioinspired catalysis

 Please wait while we load your content...
Please wait while we load your content...