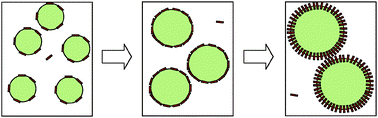
We have investigated emulsions stabilised solely by partially-hydrophobised fumed silica particles which consist of a mixture of primary particles and irregularly-shaped fused aggregates and larger agglomerates. The particle wettability is controlled by varying the extent of hydrophobisation of their surfaces. This, in turn, controls the contact angle between the oil–water interface and the particle surface (θow) which affects the particle adsorption energy and the type of emulsion formed (oil-in-water, o/w or water-in-oil, w/o). Progressive particle hydrophobisation causes transitional phase inversion of the emulsions from o/w to w/o which occurs when θow = 90° and the energy of particle adsorption to the oil–water interface is maximally favourable. The key problem addressed here is to understand why the emulsion drop size passes through a minimum at the point of emulsion phase inversion. In principle, this effect could be the result of particle desorption, changes in the extent of close-packing of the adsorbed particle film (at constant particle orientation), particle re-orientation or a combination of these processes. Using measurements of emulsion drop size and the extent of particle desorption, we have derived adsorbed particle surface concentrations as a function of the energy of desorption of the particles from the oil–water interface for a range of particle concentrations and different oil–water systems. The main conclusion is that the minimum in emulsion drop size through phase inversion is mainly caused by re-orientation of the particles from a high surface area orientation when the energy of desorption is high to a low surface area orientation when the energy of desorption is low. Some particle desorption occurs but this is a secondary effect.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...