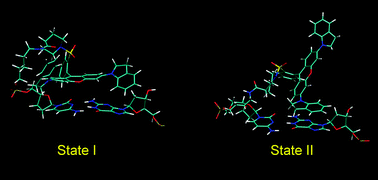
Molecular dynamics (MD) simulations and ab initio quantum chemical calculations were employed to investigate the structure, dynamics and interactions of the QSY 21 nonfluorescent quencher and the fluorescence dye Rhodamine 6G bound to a B-DNA decamer. For QSY 21, two binding motifs were observed. In the first motif, the central xanthene ring is stacked on one base of the adjacent cytosine–guanine DNA base pair, whereas one of the 2,3-dihydro-1-indolyl aromatic side rings is stacked on the other base. In the second motif, the QSY 21 stacking interaction with the DNA base pair is mediated only by one of the side rings. Several transitions between the motifs are observed during a MD simulation. The ab initio calculations show that none of these motifs is energetically preferred. Two binding motifs were found also for Rhodamine 6G, with the xanthene ring stacked predominantly either on the cytosine or on the guanine. These results suggest that the side rings of QSY 21 play a crucial role in its stacking on the DNA and indicate novel binding mode absent in the case of Rhodamine 6G, which lacks aromatic side rings.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...