TiO2 doping by hydroxyurea at the nucleation stage: towards a new photocatalyst in the visible spectral range
Abstract
We report an original method of preparation of OCN-doped TiO2 for photocatalysis in the visible spectral range. The preparation is achieved by a sol–gel route using ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Ti bond. The
O⋯Ti bond. The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond and a strengthening of the C–N and N–O bonds.
O double bond and a strengthening of the C–N and N–O bonds.
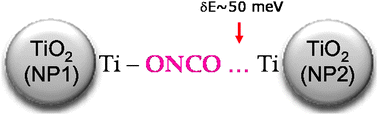

 Please wait while we load your content...
Please wait while we load your content...