Engineering of an enantioselective tyrosine aminomutase by mutation of a single active site residue in phenylalanine aminomutase†‡
Abstract
By replacing a single active-site residue
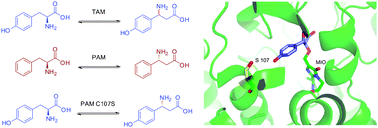
- This article is part of the themed collection: Enzymes and Proteins

 Please wait while we load your content...
Please wait while we load your content...