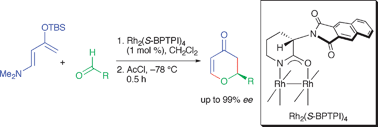
Highly enantioselective hetero-Diels–Alder reactions between Rawal’s diene and aldehydes catalyzed by chiral dirhodium(ii) carboxamidates†‡
Abstract
The first example of a chiral
- This article is part of the themed collection: Selective Catalysis for Organic Synthesis

 Please wait while we load your content...
Please wait while we load your content...