The mechanism of protonic relaxation is shown to take place in molecular systems containing hydrogen bonds. The mechanism arises from the proton redistribution between two stable states on hydrogen bond lines. This redistribution occurs due to changes of hydrogen bond double well potential, brought about by changes of the electronic state of a molecular system. A characteristic of the relaxation process is that it takes place due to the proton tunneling along hydrogen bonds. The charge shift causes electrostatic potential variation in the electron localization area, which leads to the shift of molecular system energy levels and changes its redox potential. The characteristic time of the protonic relaxation is shown to depend essentially on hydrogen bond bending strain, which increases with the temperature rise and decreases abruptly the efficiency of proton redistribution. Hence, the rate of this process decreases with temperature, in contrast to the activation process. This relaxation process is shown to be responsible for energetic characteristics of recombination reaction P+QA−→PQA (free energy difference ΔG and/or reorganization energy λ), temperature dependence in Rhodobacter sphaeroides RC.
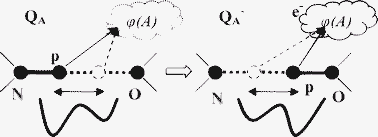
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
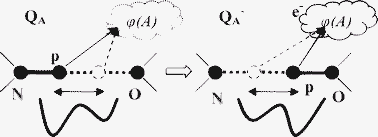

 Please wait while we load your content...
Please wait while we load your content...