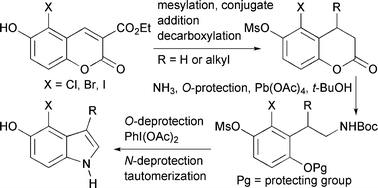
Readily accessible 3-alkoxycarbonyl-6-hydroxy-5-halocoumarins can be converted into 4-halo-5-hydroxyindoles by a sequence whose essential steps are conjugate reduction or conjugate addition, decarboxylation, lactone opening with ammonia, phenolic oxygen protection, Hofmann rearrangement to an N-Boc ethylamine, oxidation to a quinone and deprotection of the nitrogen. The resulting β-aminoethyl quinone cyclizes to a mixture of quinone imine and indole, and the imine tautomerizes to the indole spontaneously or on treatment with rhodium on alumina.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...