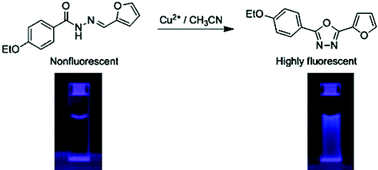
A series of N-acylhydrazones were synthesised and found to be “turn-on” fluorescent chemodosimeters for Cu2+. Among the tested transition metal ions such as Cu2+, Pb2+, Zn2+, Cd2+, Hg2+, and Ni2+, a prominent fluorescence enhancement of up to 1000-fold was only observed for Cu2+ in acetonitrile (CH3CN). This was indicated by an onset of unprecedented structured emission. Detailed experiments established that the highly Cu2+ selective fluorescence enhancement resulted from an oxidative cyclization by Cu2+of the originally nonfluorescent N-acylhydrazones into highly fluorescent rigid 1,3,4-oxadiazoles, n-dope type blocks in optoelectronic materials. The chemodosimeters can be applied to sense Cu2+ at nM levels in CH3CN and sub-µM levels in neutral aqueous environments, despite a slower response in the latter case. It is expected that these redox-based chemodosimeters might be of general applicability.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...