Intramolecular hydrogen bonding as a determinant of the inhibitory potency of N-unsubstituted imidazole derivatives towards mammalian hemoproteins†
Abstract
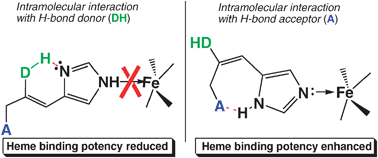
Enzymes involved in the mammalian microsomal
Maintenance work is planned for Wednesday 1st May 2024 from 9:00am to 11:00am (BST).
During this time, the performance of our website may be affected - searches may run slowly and some pages may be temporarily unavailable. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.
* Corresponding authors
a
URA 2096 du CNRS, Institut de Biologie et Technologies de Saclay, Commissariat à l’Energie Atomique, Bâtiment 528, CEA-Saclay, Gif-sur-Yvette, France
E-mail:
marcel.delaforge@cea.fr
Fax: +33 1 69 08 87 17
Tel: +33 1 69 08 68 39
b INSERM U620; Université de Rennes 1, IFR 140, 2 Avenue du Pr. Léon Bernard, Rennes, France
c
Laboratoire de Chimie Bioorganique et Bioinorganique, CNRS, Institut de Chimie Moléculaire et des Matériaux d’Orsay, UMR 8182, Bâtiment 420, Université Paris-Sud XI, Orsay, France
E-mail:
jpmahy@icmo.u-psud.fr
Fax: +33 1 69 15 72 81
Tel: +33 1 69 15 74 21
d Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, USA
Enzymes involved in the mammalian microsomal

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
