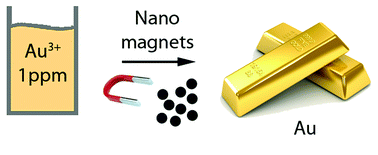
Gold adsorption on the carbon surface of C/Co nanoparticles allows magnetic extraction from extremely diluted aqueous solutions†
Abstract
The elusive chemistry of gold has made refining from ores a difficult task and often involves handling of large volumes of

 Please wait while we load your content...
Please wait while we load your content...