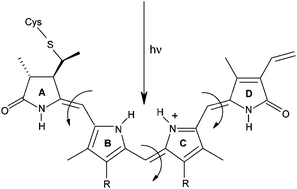
Phytochromes are widespread photoreceptors responsive to red and far-red light that exist in two photochromic forms Pr (inactive) and Pfr (active). The Pr → Pfr conversion proceeds through a series of events initiated by Z→E photoisomerization of the tetrapyrrole chromophore, believed to occur at C15 of the methine bridge between rings C and D. Recent crystal structures show that ring D in Pr is less tightly packed by the protein than rings A, B and C, which should favor the C15 reaction over reactions at C4 (AB methine bridge) and C10 (BC). In the present work, quantum chemical methods are used to establish the intrinsic reactivity of the chromophore towards all three possible Z→E isomerization events in the absence of steric effects and specific interactions with the protein. Using a level of theory that reproduces spectroscopic data with an accuracy of ∼0.2 eV, it is demonstrated that isolated conditions allow the C10 photoreaction to substantially dominate. This finding suggests that the different degrees of ring-packing observed in the protein are crucial not only to facilitate a reaction at C15, but also to prevent an intrinsically more favorable reaction at C10 from taking place.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...