Structure and magnetism of a verdazyl radical clathrate hydrate. Strong intermolecular magnetic interactions derived from π-stacking within ice-like channels†
Abstract
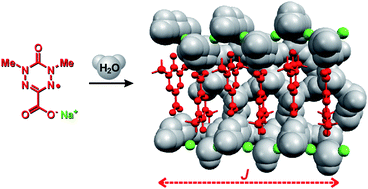
Deprotonation of 1,5-dimethyl-6-oxoverdazyl-3-carboxylic acid (2H) in
- This article is part of the themed collection: Crystal engineering in molecular magnetism

 Please wait while we load your content...
Please wait while we load your content...