Selection between block- and homo-polyelectrolytes through polyion complex formation in aqueous medium
Abstract
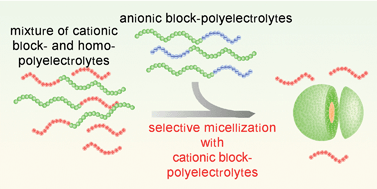
A strict selection of an oppositely charged pair of block-polyelectrolytes, consisting of ionic and non-ionic hydrophilic blocks, was found to occur in aqueous medium upon the addition of poly(ethylene glycol)-b-poly(α,β-aspartic acid) [PEG–P(Asp)] (anionic block-polyelectrolyte) solution into a mixed solution of PEG-b-poly(L-lysine) [PEG–P(Lys)] (cationic block-polyelectrolyte) and P(Lys) (homo-polyelectrolyte), leading to the formation of polyion complex (PIC) micelles exclusively composed of block-polyelectrolytes pairs. The selection process seems to proceed under the condition of dynamic equilibrium, because the addition of a stoichiometric amount of PEG–P(Lys) to a solution of PIC micelles composed of PEG–P(Asp) and P(Lys) resulted in nearly complete (96%) replacement of P(Lys) with PEG–P(Lys) in the micelles. This phenomenon may be explained by assuming the increased association force of block-polyelectrolytes compared with homo-polyelectrolyte, presumably due to the frustrated conformation of block-polyelectrolytes composed of incompatible segments, such as PEG and polyelectrolyte, in aqueous medium. An increase in the association force of block-polyelectrolytes was also supported by the formation of non-stoichiometric PIC associates in the range in which the block-polyelectrolyte comprises a major component in the medium ([Lys] < [Asp]); in this range, all of the PEG–P(Asp) strands behave as host molecules to participate in the complexation with P(Lys) strands as guest molecules, leaving no free PEG–P(Asp) strands in the medium. Alternatively, P(Lys) strands seem to always behave as guest molecules to form stoichiometric PIC micelles with PEG–P(Asp), even when they exist as a major component ([Lys] > [Asp]), leaving excess P(Lys) strands in the free form in the medium. This new mode of molecular recognition based on

 Please wait while we load your content...
Please wait while we load your content...