Synthetic and biological studies on the spiro-mamakone system†
Abstract
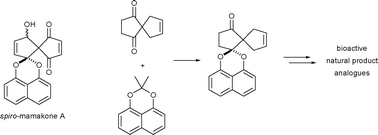
An exploration of the chemistry of the spiro-mamakone system, exemplified by the cytotoxic, fungal metabolitespiro-mamakone A, is presented. The first reported synthesis of the spiro-mamakone carbon skeleton was achieved, as well as the synthesis of a variety of closely related analogues of the natural product. Biological testing of the synthetic analogues generated a structure–activity profile for the natural product, establishing the importance of the enedione moiety to biological activity.

 Please wait while we load your content...
Please wait while we load your content...