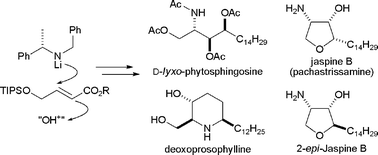
The highly diastereoselective anti-aminohydroxylation of (E)-γ-tri-iso-propylsilyloxy-α,β-unsaturated esters, viaconjugate addition of lithium (S)-N-benzyl-N-(α-methylbenzyl)amide and subsequent in situ enolate oxidation with (+)-(camphorsulfonyl)oxaziridine, has been used as the key step in the asymmetric synthesis of N,O,O,O-tetra-acetyl D-lyxo-phytosphingosine (20% yield over 7 steps), the anhydrophytosphingosine jaspine B (10% yield over 9 steps), 2-epi-jaspine B (14% yield over 9 steps), and the Prosopisalkaloid deoxoprosophylline (26% yield over 7 steps).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...