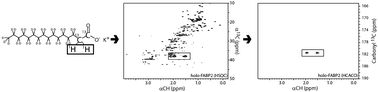
Here we describe the synthesis and evaluation of a new isotopic labeling strategy for fatty acids to be used as probes for studying ligand binding by NMR. We synthesized palmitic acid with carbons C-3 through C-16 perdeuterated, C-1 and C-2 with 13C atoms and hydrogens at C-2. Our strategy began with commercially available perdeuterated myristic acid and built up to palmitic acid using a Horner–Wadsworth–Emmons reaction. To evaluate the power of this isotopic enrichment strategy, we evaluated ligand binding to a prototypical member of the intracellular lipid binding protein family, FABP2. This small 15 kDa protein is well known to bind fatty acids with high affinity, and we used this system to illustrate the spectral filtering abilities of our described labeling strategy. Herein we show how having two vicinal 13C-enriched carbons, two hydrogens at the α-position, and a perdeuterated aliphatic tail allows the efficient use of multidimensional NMR experiments to effectively filter all background resonances from the protein and facilitate the study of ligand binding.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...