Thermo-solvatochromism of chloro-nickel complexes in 1-hydroxyalkyl-3-methyl-imidazolium cation based ionic liquids
Abstract
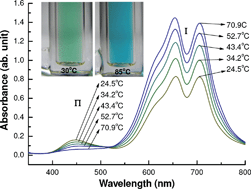
Sunlight can be directly absorbed by many coloured solids or liquids to re-generate heat but the temperature achievable is usually below 100 °C. Consequently, thermally responsive physical and/or chemical processes that can effectively utilise this almost free but low temperature solar heat are becoming increasingly important, considering the inevitable change in energy supply from fossil fuels to renewable sources in the near future. In this work, the thermochromic and solvatochromic behaviour of chloro-Ni(II) complexes was investigated by visual observation and vis-spectroscopy in 1-hydroxyalkyl-3-methylimidazolium (CnOHmim+, n = 2 or 3) based ionic liquids between room temperature and 85 °C. The thermochromism was a result of the tetrahedral complex, NiCl42− (blue, hot) being solvolysed into various octahedral complexes, e.g. [NiClx(CnOHmim+–ClO4−)y]2−x (x + y = 6) or [NiClx(CnOHmim)y]z+–(ClO4−)z (z = 2 + y − x) (yellow or green, cold) in the ionic liquids. The capability of the CnOHmim+ ligand to encourage the formation of octahedral chloro-Ni(II) complexes with a high number of chloride ligands could be attributed to the electrostatic attraction in the octahedral configurations. These new systems were found to be sensitive to

 Please wait while we load your content...
Please wait while we load your content...