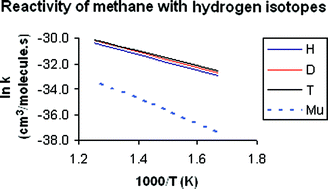
The rate constants and kinetic isotope effects of the reaction of methane with four isotopes of hydrogen, protium (H), deuterium (D), tritium (T), and muonium (Mu), were studied using variational transition state theory with multidimensional tunneling on an analytical potential energy surface, PES-2002, previously constructed by our group. For the four isotopes, our kinetics results agree reasonably with available experimental measurements, improving previous theoretical results that used different potential energy surfaces and/or theoretical approaches. In the comparison of the reactivity between protium and muonium, which is the most severe test of the surface and theoretical method due to the large mass difference between the two isotopes, some sources of discrepancy between theory and experiment were analyzed. These were the zero-point energy, tunneling effect, and the role of the reactivity from methane excited vibrational states.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...