Mechanistic evidence for remote π-aryl participation in acid-catalyzed ring opening of homobenzoquinone epoxides†
Abstract
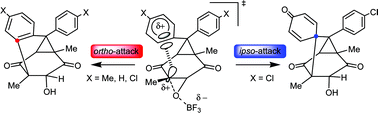
The acid-induced reaction of bis(p-chlorophenyl)homobenzoquinone epoxide gave the dual ipso/ortho intramolecular SE2-Ar products associated with π-aryl participated oxirane ring opening, whereas bis(p-tolyl)- and diphenyl-substituted homologues provided only the ortho products.

 Please wait while we load your content...
Please wait while we load your content...