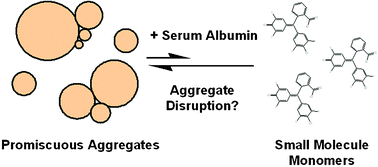
At micromolar concentrations, many molecules form aggregates in aqueous solution. In this form, they inhibit enzymes non-specifically leading to false positive “hits” in enzyme assays, especially when screened in high-throughput. This inhibition can be attenuated by bovine serum albumin (BSA); the mechanism of this effect is not understood. Here we present evidence that BSA, lysozyme, and trypsin prevent inhibition when incubated at milligram per millilitre concentrations with aggregates prior to the addition of the monitored enzyme. These solutions still contained aggregates by dynamic light scattering (DLS), suggesting that inhibition is prevented by saturating the aggregate, rather than disrupting it. For most combinations of aggregate and protein, inhibition was not reversed if the competing protein was added after the incubation of aggregates with the monitored enzyme. In the one exception where modest reversal was observed, DLS and flow cytometry indicated that the effect was due to the disruption of aggregates. These results suggest that aggregate-bound enzyme is not in dynamic equilibrium with free enzyme and that bound enzyme cannot be displaced by a competing protein. To further test this hypothesis, we incubated aggregate-bound enzyme with a specific, irreversible inhibitor and then disrupted the aggregates with detergent. Most enzyme activity was restored on aggregate disruption, indicating no modification by the irreversible inhibitor. These results suggest that enzyme is bound to aggregate so tightly as to prevent any noticeable dissociation and that furthermore, aggregates are stable at physiologically relevant concentrations of protein.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...