Fabrication of a nanoparticle gradient substrate by thermochemical manipulation of an ester functionalized SAM†
Abstract
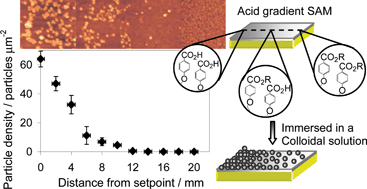
The hydrolysis of methyl ester (–CO2Me) and tert-butyl ester (–CO2tBu) functionalized SAMs as a function of subphase temperature and pH is described. Contact angle measurements show that the methyl ester functionalized monolayer does not hydrolyse in pH 1–13 aqueous solutions heated up to 80 °C. In contrast, the –CO2tBu functionalized monolayer hydrolysed below pH 5. The rate and the extent of the hydrolysis were dependent on the temperature and pH of the aqueous solution. Using the Cassie equation, the activation energy for the hydrolysis of CO2tBu-phenyl functionalized SAM was determined as 75 ± 7 kJ mol−1 from the contact angle measurements. Furthermore, the adhesion properties of –CO2tBu and –COOH functionalized SAMs were investigated by depositing –NR2 and –COOH functionalized polystyrene nanoparticles onto the surfaces at pH 3 and 9. By AFM, it was observed that the particles bind preferentially to the –COOH functionalized SAM and the adhesion was pH dependent, with the largest coverage being observed at pH 3. Using the acquired understanding of the hydrolysis of –CO2tBu functionalized SAM and the particle adhesion properties, a simple and facile approach towards fabricating a particle density gradient on this surface is demonstrated. An acid gradient SAM (20 mm long) was prepared by mounting one end of a –CO2tBu functionalized SAM onto the hot side of a Peltier element (80 °C) in pH 1 aqueous solution. The substrate was subsequently immersed into a colloidal solution of –NR2 functionalized polystyrene nanoparticles, removed and rinsed. By AFM, the particle density was shown to be dependent on the surface coverage of –COOH moieties of the underlying SAM. The density started at 104 particles µm−2 on the hydrolysed end down to 0 particles µm−2 on the non-hydrolysed end.

 Please wait while we load your content...
Please wait while we load your content...