The canonical and other mechanisms of elementary chemical reactions†
Abstract
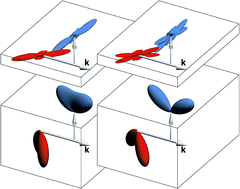
This article introduces a definition of the concept of elementary reaction mechanism that, while conforming to the traditional view of reaction mechanisms as dynamical processes whereby reagents are transformed into products, sharpens it by requiring reagent and product states to be completely specified and fully correlated. This leads to well-defined mathematical requirements for classification of a dynamical process as a reaction mechanism and also to a straightforward mathematical procedure for the determination of a special class of independent collision mechanisms that are dubbed “canonical”. Canonical mechanisms result from an exact decomposition of the differential cross section of the reaction and form a complete orthogonal basis in terms of which all reaction mechanisms can be described. Examples involving the benchmark F + H2 and D + H2 reactions at energies ranging from ultralow to hyperthermal illustrate how canonical and other reaction mechanisms can be visualised and also how analysis of a reaction in terms of its canonical mechanisms can provide insight into its dynamics.

 Please wait while we load your content...
Please wait while we load your content...