Abstract
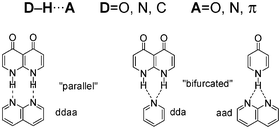
The nature of hydrogen bond interactions (HB) is still today the subject of many discussions. We present an overview of computational methods and parameters (interaction energy, HB distance and radii, electron density topological parameters or orbital energies) required for an accurate description of HB systems. As well, we present the different correlations that have been found between these descriptors providing a global view of HB interactions. A synopsis of the different HBs reported in terms of their strength was presented. Considering the definitions of covalent and ionic bonds, HB interactions could occur between these two extremes. Thus, we look into some of the very strong HBs (LBHB, CAHB, RAHB) and some of the weak HBs (weak donors: C–H or weak acceptors: π systems). Subsequently, aspects such as cooperativity or solvation are examined. Finally, we present a study on multiple “parallel” and “bifurcated” HB systems. Our results indicate that HB pattern and electron density determine the strength of the interaction and that “parallel” HB interactions are more stable than the “bifurcated” ones.

 Please wait while we load your content...
Please wait while we load your content...