266 nm photolysis of CF3I and C2F5I studied by diode laser gain FMspectroscopy
Abstract
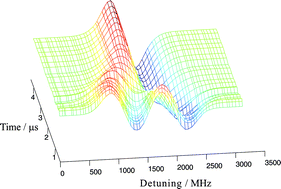
Frequency modulated diode laser based absorption at 1.315 μm has been used to measure the Doppler lineshapes of the I(2P1/2–2P3/2) transition in atomic iodine produced from the 266 nm photolysis of both CF3I and C2F5I. Wavelength resolved laser gain is seen following photolysis as excited iodine atoms (2P1/2) are produced with a quantum yield close to unity from photolysis of both parent molecules. Time resolved measurements were made and the nascent speed distribution and translational anisotropy parameter, β were determined. Mean atomic speeds of 800 and 850 ms−1, which correspond to 83 and 68% of the maximum possible kinetic energy release into the iodine photofragment, were determined for photolysis of CF3I and C2F5I, respectively. The nascent translational anisotropy parameter was found to be β = 1.77 ± 0.05 for CF3I and β = 1.69 ± 0.05 for C2F5I. These values are explicable in terms of parent rotational motion and non-adiabatic processes in the exit channel.

 Please wait while we load your content...
Please wait while we load your content...