Mid-infrared vibrational spectra of discrete acetone-ligated cerium hydroxide cations
Abstract
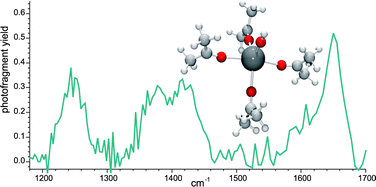
Cerium(III) hydroxy reactive sites are responsible for several important heterogeneous catalysis processes, and understanding the reaction chemistry of substrate molecules like CO, H2O, and CH3OH as they occur in heterogeneous media is a challenging task. We report here the first infrared spectra of model gas-phase cerium complexes and use the results as a benchmark to assist evaluation of the accuracy of ab initio calculations. Complexes containing [CeOH]2+ ligated by three- and four-acetone molecules were generated by electrospray ionization and characterized using wavelength-selective infrared multiple photon dissociation (IRMPD). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency for the [CeOH(acetone)4]2+ species appeared at 1650 cm−1 and was red-shifted by 90 cm−1 compared to unligated acetone. The magnitude of this shift for the carbonyl frequency was even greater for the [CeOH(acetone)3]2+ complex: the IRMPD peak consisted of two dissociation channels, an initial elimination of acetone at 1635 cm−1, and elimination of acetone concurrent with a charge separation producing [CeO(acetone)]+ at 1599 cm−1, with the overall frequency centered at 1616 cm−1. The increasing red shift observed as the number of acetone ligands decreases from four to three is consistent with transfer of more electron density per ligand in the less coordinated complexes. The lower frequency measured for the elimination/charge separation process is likely due to a combination of: (a) anharmonicity resulting from population of higher vibrational states, and (b) absorption by the initially formed photofragment [CeOH(acetone)2]2+. The C–C stretching frequency in the complexes is also influenced by coordination to the metal: it is blue-shifted compared to bare acetone, indicating a slight strengthening of the C–C bond in the complex, with the intensity of the absorption decreasing with decreasing ligation. Density functional theory (DFT) calculations using three different functionals (VWN, B3LYP, and PBE0) were used to predict the infrared spectra of the complexes. Calculated frequencies for the carbonyl stretch are within 40 cm−1 of the IRMPD of the three-acetone complex measured using the single acetone loss, and within 60 cm−1 of the measurement for the four-acetone complexes. The B3LYP functionals provided the best agreement with the measured spectra, with the VWN modestly lower and PBE0 modestly higher. The C–C stretching frequencies calculated using B3LYP are higher in energy than the measured values by ∼30 cm−1, and reproduce the observed trend which shows that the C–C stretching frequency decreases with increasing ligation. Agreement between C–C frequency and calculation was not as good using the VWN functional, but still within 70 cm−1. The results provide an evaluation of changes in the acceptor properties of the metal center as ligands are added, and of the utility of DFT for modeling f-block coordination complexes.
O stretching frequency for the [CeOH(acetone)4]2+ species appeared at 1650 cm−1 and was red-shifted by 90 cm−1 compared to unligated acetone. The magnitude of this shift for the carbonyl frequency was even greater for the [CeOH(acetone)3]2+ complex: the IRMPD peak consisted of two dissociation channels, an initial elimination of acetone at 1635 cm−1, and elimination of acetone concurrent with a charge separation producing [CeO(acetone)]+ at 1599 cm−1, with the overall frequency centered at 1616 cm−1. The increasing red shift observed as the number of acetone ligands decreases from four to three is consistent with transfer of more electron density per ligand in the less coordinated complexes. The lower frequency measured for the elimination/charge separation process is likely due to a combination of: (a) anharmonicity resulting from population of higher vibrational states, and (b) absorption by the initially formed photofragment [CeOH(acetone)2]2+. The C–C stretching frequency in the complexes is also influenced by coordination to the metal: it is blue-shifted compared to bare acetone, indicating a slight strengthening of the C–C bond in the complex, with the intensity of the absorption decreasing with decreasing ligation. Density functional theory (DFT) calculations using three different functionals (VWN, B3LYP, and PBE0) were used to predict the infrared spectra of the complexes. Calculated frequencies for the carbonyl stretch are within 40 cm−1 of the IRMPD of the three-acetone complex measured using the single acetone loss, and within 60 cm−1 of the measurement for the four-acetone complexes. The B3LYP functionals provided the best agreement with the measured spectra, with the VWN modestly lower and PBE0 modestly higher. The C–C stretching frequencies calculated using B3LYP are higher in energy than the measured values by ∼30 cm−1, and reproduce the observed trend which shows that the C–C stretching frequency decreases with increasing ligation. Agreement between C–C frequency and calculation was not as good using the VWN functional, but still within 70 cm−1. The results provide an evaluation of changes in the acceptor properties of the metal center as ligands are added, and of the utility of DFT for modeling f-block coordination complexes.

 Please wait while we load your content...
Please wait while we load your content...