Enantioselectivity in the boron aldol reactions of methyl ketones†
Abstract
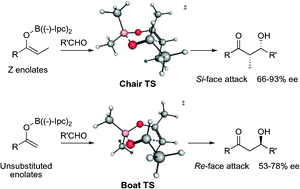
DFT computed transition states quantitatively explain the surprising stereochemical outcome of unsubstituted enolborinates in diastereoselective and enantioselective boron aldol reactions.

 Please wait while we load your content...
Please wait while we load your content...