A highly enantioselective total synthesis of (+)-goniodiol
Abstract
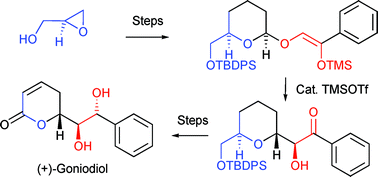
A high-yielding enantioselective total synthesis of the bioactive styryllactone (+)-goniodiol has been realised, starting from readily available (S)-glycidol. A key step is an oxygen-to-carbon rearrangement of a silyl enol ether linked via an anomeric centre, facilitating the rapid and diastereoselective construction of this functionalised system.

 Please wait while we load your content...
Please wait while we load your content...