Photochemical intramolecular aromatic substitutions of the imidazol-2-yl radical are superior to those mediated by Bu3SnH
Abstract
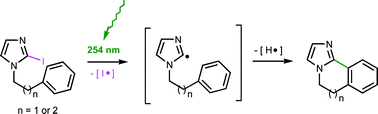
Six-membered photochemical cyclisations of 2-iodo-N-(2-arylethyl)imidazoles proceeded regioselectively in higher yields than the equivalent tin hydride-mediated reactions. The decrease in yield of cyclisation products, 5,6-dihydroimidazo[2,1-a]isoquinolines containing strongly deactivating substituents on the aryl ring confirmed the electrophilic nature of the σ-imidazol-2-yl radicals. The seven-membered cyclisation was only successful under photochemical conditions, as radical reduction occurred with tin hydride. Nitration of 5,6-dihydroimidazo[2,1-a]isoquinoline with nitric/sulfuric acid occurred at the 2- and 8-positions.

 Please wait while we load your content...
Please wait while we load your content...