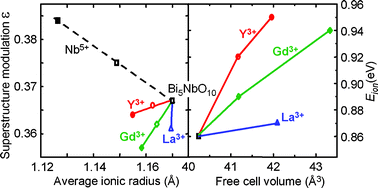
The effects of partial substitutions of Bi by La, Gd, and Y on the structure, thermal expansion and transport properties of modulated fluorite-type Bi5NbO10 were investigated using X-ray thermodiffraction, alternative current impedance spectroscopy and the Wagner polarization method. Through conventional solid-state syntheses, the solid solutions Bi5−xLnxNbO10 exist up to about x
∼ 0.25 for La, and to x
∼ 0.5 for Gd and Y. A doubling of their thermal expansion coefficient is observed above 540 °C. The type-II incommensurate modulation of these phases is correlated to the nature and amount of substitute, and disappears above 800 °C. Except for Bi5NbO10, which appears to be a pure ionic conductor, most of the solid solutions exhibit mixed conductivity with predominant ionic transport. Partial Gd and Y substitutions favor electron conductivity in the low temperature range, probably due to bismuth partial oxidation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...