Breaking the N2 triple bond: insights into the nitrogenase mechanism
Abstract
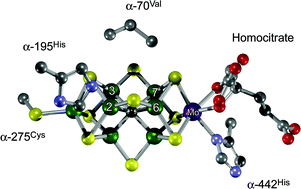
Nitrogenase is the metalloenzyme that performs biological nitrogen fixation by catalyzing the reduction of N2 to ammonia. Understanding how the nitrogenase active site metal cofactor (FeMo-cofactor) catalyzes the cleavage of the N2 triple bond has been the focus of intense study for more than 50 years. Goals have included the determination of where and how substrates interact with the FeMo-cofactor, and the nature of reaction intermediates along the reduction pathway. Progress has included the trapping of intermediates formed during turnover of non-physiological substrates (e.g., alkynes, CS2) providing insights into how these molecules interact with the nitrogenase FeMo-cofactor active site. More recently, substrate-derived species have been trapped at high concentrations during the reduction of N2, a diazene, and hydrazine, providing the first insights into binding modes and possible mechanisms for N2 reduction. A comparison of the current state of knowledge of the trapped species arising from non-physiological substrates and nitrogenous substrates is beginning to reveal some of the intricacies of how nitrogenase breaks the N2 triple bond.

 Please wait while we load your content...
Please wait while we load your content...