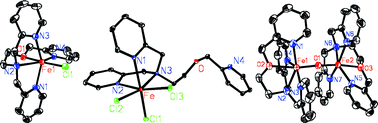
Three mononuclear iron complexes and one binuclear iron complex, [Fe(tpoen)Cl]·0.5(Fe2OCl6) (1), [Fe(tpoen)Cl]PF6 (2), Fe(tpoen)Cl3 (3) and [{Fe(tpoen)}2(µ-O)](ClO4)4 (4) (tpoen = N-(2-pyridylmethoxyethyl)-N,N-bis(2-pyridylmethyl)amine), were synthesized as functional models of non-heme iron oxygenases. Crystallographic studies revealed that the Fe(II) center of 1 is in a pseudooctahedral environment with a pentadentate N4O ligand and a chloride ion trans to the oxygen atom. The Fe(III) center of 3 is ligated by three nitrogen atoms of tpoen and three chloride ions in a facial configuration. Each Fe(III) center of 4 is coordinated with four nitrogen atoms and an oxygen atom of tpoen with the Fe–O–Fe angle of 172.0(3) Å. Complexes 2, 3 and 4 catalysed the oxidation of cyclohexane with H2O2 in the total TNs of 24–36 with A/K ratios of 1.9–2.4. Under the same conditions they also catalysed both the oxidation of ethylbenzene to benzylic alcohol and acetobenzene with good activity (30–47 TN) and low selectivity (A/K 0.7), and the oxidation of adamantane with moderate activity (15–18 TN) and low regioselectivity (3°/2° 3.0–3.2). With mCPBA as oxidant the catalytic activities of 2, 3 and 4 increased 1.8 to 2.3-fold for the oxidation of cyclohexane and ethylbenzene and 6.3 to 7.5-fold for the oxidation of adamantane. Drastic enhancement of the regioselectivity was observed in the oxidation of adamantane (3°/2° 18.5–30.3).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...